When dealing with sterile pharmaceutical productions there are two main aspects to be considered: products’ protection and operators’ safety.
It is therefore of utter importance to minimize human intervention to reduce the risk of product contamination and guarantee the highest protection towards the operators.
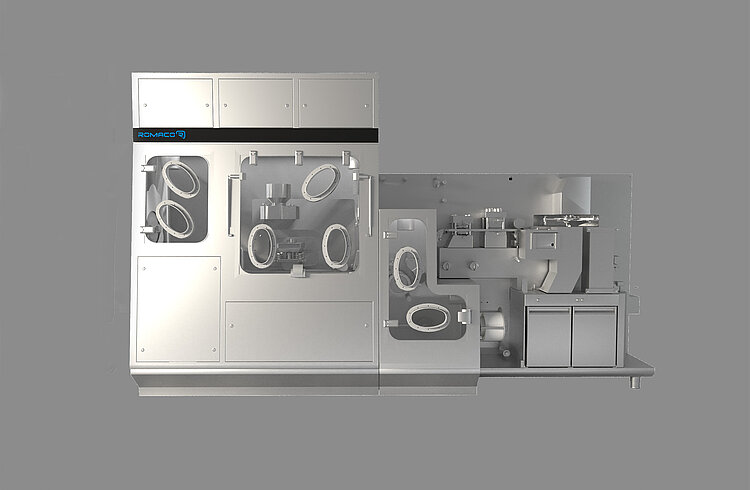
The isolator, being a physical barrier, allows the implementation of a successful contamination control strategy, with full automated process and monitoring system, as well as minimum human intervention.

Product sterility
Operators’ safety
Contamination
Process control
Production efficiency
Bio-decontamination
Assembly of parts and loading of components in line with the aseptic process
Maintenance costs
Downtime
Energy consumption
Stringent guidelines
Validation
Data integrity
High grade stainless steel isolator, fully integrated on machine base
GMP Grade A environment (ISO 4.8)
Laminar Air Flow
Dedicated AHU
Pressure management
Air tightness compliant to Class III (according to ISO 10648:2)
Automatic leak test (including bellows leak test)
Integrated WIP/CIP systems
Bi-Bo filter units
Inlet & Outlet HEPA 14 filters
Enhanced ergonomics
Containment up to OEB 5
Energy-saving design
Optimized automatic bio-decontamination systems (VPHP and aHP)
Aseptic transfer systems
Full compliance to GAMP 5, 21 CFR part 11
Romaco isolators allow the implementation of a successful contamination control strategy, with full automated process and monitoring systems, minimizing human intervention and guaranteeing operators’ safety.