When handling HPAPI or cytotoxic products, as disciplined in the latest Annex 1 release, the use of appropriate Barrier technologies should be taken into consideration.
In order to ensure necessary conditions and reduce microbiological contamination linked to direct human interventions in the production area, isolators are the kind of technology the pharmaceutical industry is relying onto.
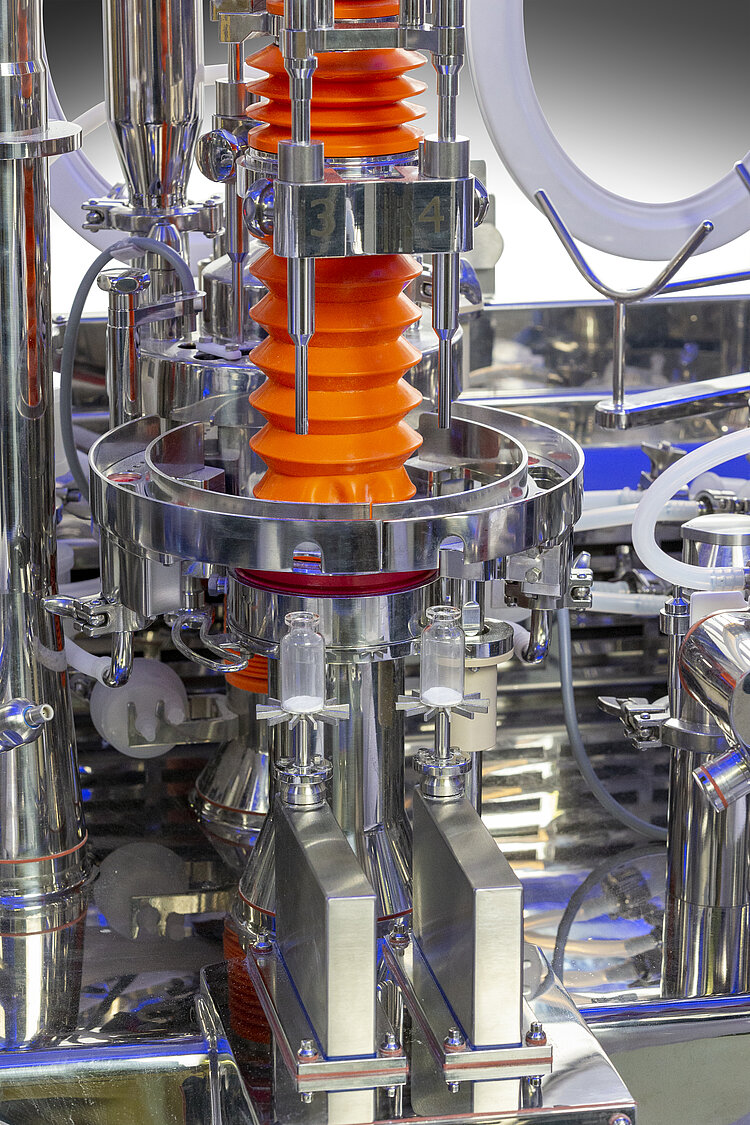
There are nevertheless many zones of potential contamination in any isolator, which should be assessed in the Risk Analysis and in the Contamination Control Strategy.

Preventing product contamination
Ensuring operators’ safety
Implementing an effective contamination control strategy
Gloves integrity tests…but not only!
Validating the process
Thoroughly cleaning the system
Loading of components
Assembling and disassembling the machine units in sterile conditions
6-walls isolation design, up to OEB 5, including safe transfer of all components
Enhanced cleaning capability thanks to:
Automatic CIP/WIP
FlatSeal™inflatable gasket proprietary design
Belleak™ technology:
Active Breathing functionality, Internal Pressure SetPoint in order to maintain negative throughout the cycles
Pressure decay integrity test on bellows
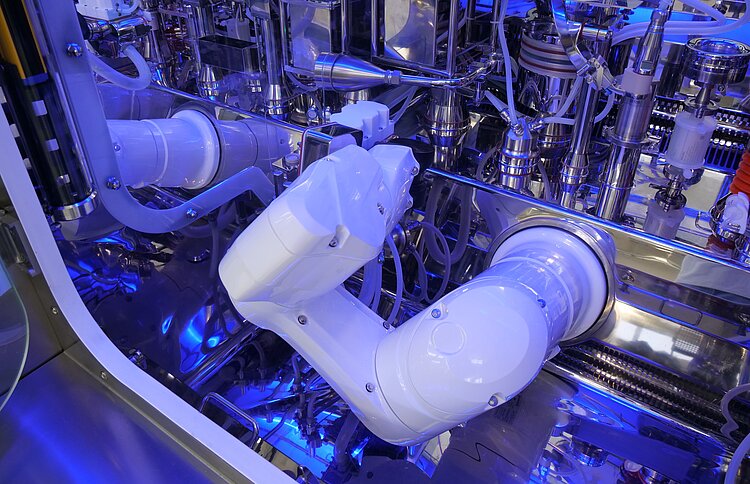
We are ready to bring your production to the next level of contamination control.